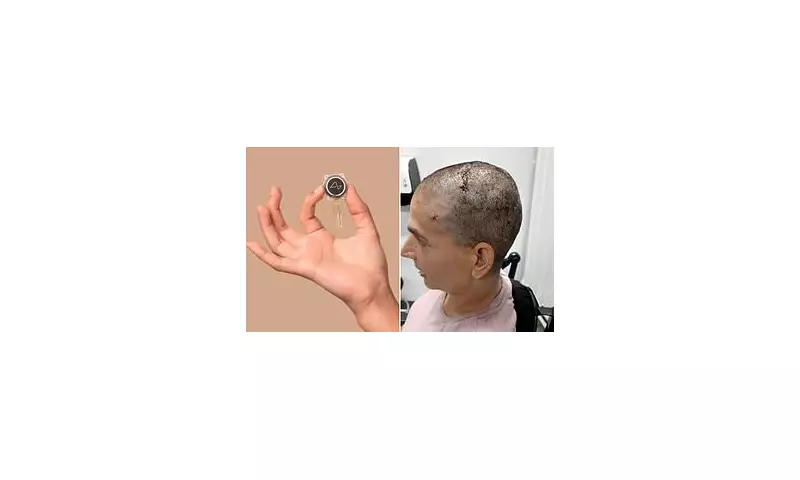
Elon Musk's ambitious neurotechnology venture, Neuralink, is preparing to launch groundbreaking brain-chip trials in the UK. The company, which recently received regulatory approval, aims to test its implantable devices on British patients suffering from severe neurological conditions.
How Neuralink's Brain Chips Work
The coin-sized Neuralink device is designed to be implanted directly into the brain, where it can both record neural activity and stimulate specific regions. The technology could potentially:
- Restore mobility for paralysis patients
- Treat Parkinson's disease and epilepsy
- Help blind people see and deaf people hear
UK at the Forefront of Neurotech Innovation
Britain has been selected as one of the first countries outside the US to host human trials, thanks to its robust medical research infrastructure. The UK's Medicines and Healthcare products Regulatory Agency (MHRA) has given the green light for preliminary testing.
Ethical Concerns and Public Debate
While the potential medical benefits are significant, the technology has sparked intense debate:
- Privacy concerns about brain data collection
- Questions about long-term safety
- Fears of human enhancement creating societal divides
Neuralink maintains that its current focus is strictly medical, with no plans for consumer applications in the near future.





