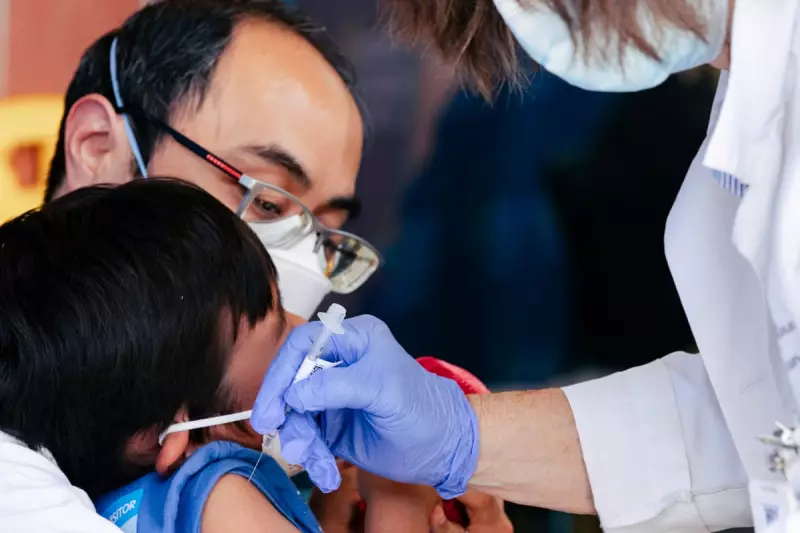
The US Food and Drug Administration (FDA) has taken a significant step in the fight against COVID-19 by approving Pfizer's vaccine for children aged 5 to 11. This decision comes after extensive clinical trials demonstrated the vaccine's safety and efficacy in younger age groups.
A Milestone in Paediatric Healthcare
The authorisation represents a crucial development in protecting children from COVID-19, particularly as schools reopen and colder months approach. Health experts anticipate this move will help reduce transmission rates and prevent severe illness in younger populations.
Key Details About the Approval
- The vaccine dosage for children is one-third of the adult dose
- Clinical trials showed 90.7% efficacy in preventing symptomatic COVID-19
- Side effects were generally mild and comparable to other routine childhood vaccinations
What This Means for Families
With this approval, approximately 28 million American children become eligible for vaccination. Public health officials are urging parents to consider vaccinating their children to help achieve broader community immunity.
The Centers for Disease Control and Prevention (CDC) will now review the FDA's decision before vaccinations can officially begin. If approved, rollout could start within weeks.





