Celtic Curse Genetic Hotspots Uncovered in Pioneering UK and Ireland Study
Researchers have unveiled the specific regions across the United Kingdom and Ireland that serve as hotspots for the so-called Celtic Curse. This condition, medically known as haemochromatosis, is a relatively obscure genetic disorder characterised by an excessive accumulation of iron in the bloodstream. If not addressed promptly, haemochromatosis can precipitate severe health complications, including liver damage, diabetes, arthritis, and cardiac issues.
Groundbreaking Mapping of Genetic Prevalence
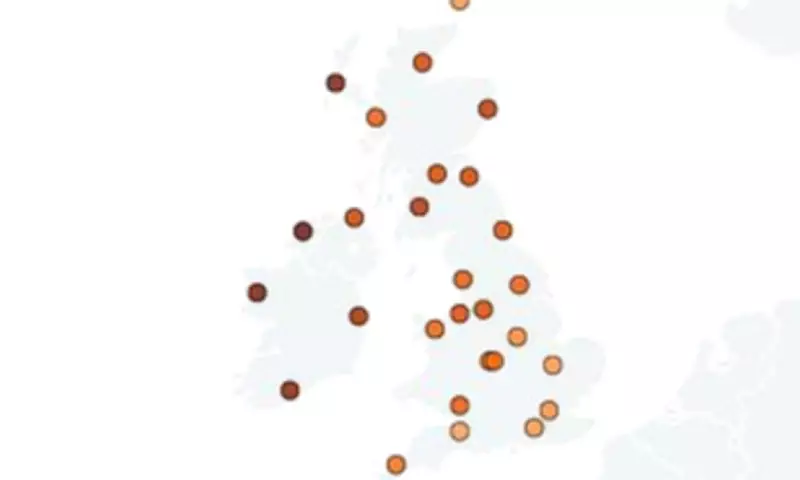
For the first time, a team from the University of Edinburgh has meticulously charted the distribution of this condition throughout the UK and Ireland. Their findings robustly affirm the aptness of its Celtic moniker. By scrutinising genetic data from more than 400,000 participants in the UK BioBank and Viking Genes studies, the scientists pinpointed where the C282Y genetic variant, the primary driver of the disease, is most frequently encountered.
The analysis disclosed that individuals with ancestral ties to the northwest of Ireland face the greatest likelihood of developing the Celtic Curse. Remarkably, approximately one in every 54 people from this area is estimated to carry the C282Y variant. This stands in stark contrast to regions like the southwest of England, where the prevalence drops to just one in 218.
Regional Risk Variations and Historical Insights
The study, published in the esteemed journal Nature Communications, further identified the Outer Hebrides as the second-highest risk area, with one in 62 residents carrying the gene. Northern Ireland follows closely, with a rate of one in 71. Mainland Scots, particularly those in Glasgow and southwest Scotland, also exhibit a significantly elevated risk, with one in 171 individuals affected.
Intriguingly, the research examined haemochromatosis diagnoses within NHS England, uncovering over 70,000 cases. Diagnoses were nearly four times higher among white Irish patients compared to their white British counterparts. A striking example is Liverpool, where residents are 11 times more likely to receive a diagnosis than those in Kent. This disparity is attributed to Liverpool's historical influx of Irish immigrants during the 1850s, when about 20% of the city's population was of Irish descent, thereby amplifying the gene's prevalence.
Calls for Genetic Screening and Early Intervention
Co-author Professor Jim Wilson posits that the variant likely originated in a Scottish or Irish individual around 5,000 years ago. He emphasises the critical need for early detection, as symptoms can take decades to manifest, often leading to irreversible organ damage. Regular blood donation is a primary treatment to mitigate long-term harm.
Professor Wilson advocates for a genetic screening programme in high-risk areas like the Western Isles and Northern Ireland. The Western Isles, with a population under 30,000, present an ideal test bed for such initiatives, he notes, with plans underway to launch a fundraising campaign for a screening pilot.
Echoing this sentiment, Torcuil Crichton, Labour MP for the Western Isles and a haemochromatosis patient, urges ministers to reconsider community-wide screening. Early identification, he asserts, can prevent a host of adverse health outcomes, underscoring the importance of proactive measures.
Understanding Haemochromatosis: Symptoms and Management
Haemochromatosis disrupts the body's iron regulation, leading to a dangerous buildup. Symptoms may include persistent fatigue, weight loss, joint pain, and cognitive issues like brain fog. Management typically involves regular blood removal via venesection or donation, initially weekly and then periodically, alongside dietary adjustments such as avoiding iron-fortified cereals and excessive alcohol.
While carrying the C282Y gene does not guarantee development of the condition, having two copies raises the risk to 56%, compared to zero for those without the gene. This highlights the urgency of awareness and screening in identified hotspots to safeguard public health.





