Charities Launch Formal Appeal Against NHS Cancer Treatment Decision
Three prominent charities have initiated a formal appeal process against the National Institute for Health and Care Excellence's (Nice) recent recommendation to withdraw a potentially life-saving cancer therapy from NHS availability in England and Wales. Blood Cancer UK, Lymphoma Action and the stem cell charity Anthony Nolan have jointly submitted their appeal, expressing significant concerns for patients facing limited treatment alternatives.
Treatment at the Centre of the Controversy
Tecartus, also known scientifically as brexu-cel, represents a cutting-edge CAR T-cell therapy that engineers a patient's own immune cells to target and destroy cancer cells. The treatment had been accessible through the Cancer Drugs Fund for individuals diagnosed with a rare form of non-Hodgkin lymphoma called mantle cell lymphoma, while additional long-term efficacy data was being gathered. This blood cancer affects approximately 600 people annually across the United Kingdom, primarily impacting the white blood cells crucial for fighting infections.
Nice's independent committee, after reviewing nearly five years of real-world data from NHS usage alongside clinical trial results, concluded that the therapy's performance in everyday clinical practice did not match the outcomes observed during controlled trials. The watchdog reported median survival rates of 2.5 years for NHS patients compared to four years in the original research studies.
Charity Concerns and Patient Impact
Dr Rubina Ahmed, serving as director of research, policy and services at Blood Cancer UK, articulated the organisation's position clearly. "For some people with mantle cell lymphoma, whose cancer has returned or proven resistant to previous treatments, this CAR T-cell therapy represents a final hope for a cure," she stated. "While we acknowledge that Nice decisions involve intricate clinical and economic evaluations, we remain deeply concerned about the implications for patients with severely limited alternatives. Our formal appeal raises important questions regarding the assessment methodology and the practical consequences of this decision for affected individuals."
Emily John, a specialist nurse at Anthony Nolan with direct experience administering the treatment, expressed profound disappointment. "Having witnessed firsthand how Tecartus has transformed the lives of patients living with mantle cell lymphoma, offering them a crucial lifeline when other options failed, this decision feels particularly devastating," she explained. "The removal of the only CAR T-cell therapy available for this specific condition represents a troubling regression in NHS care standards. We strongly urge Nice and the pharmaceutical manufacturer to collaborate on finding a viable solution that preserves access to this potentially life-saving treatment."
Patient Testimony Highlights Treatment Value
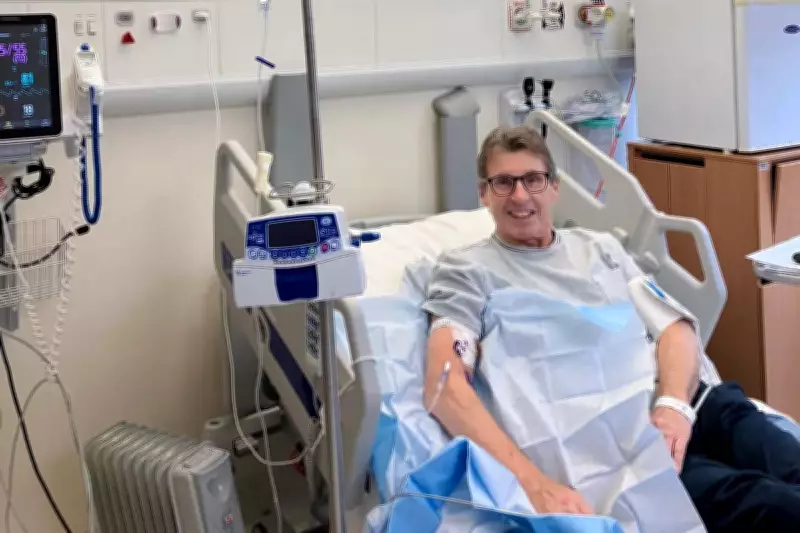
The human impact of this decision is powerfully illustrated through patient experiences. Paul Madley, a 66-year-old chartered surveyor from Cardiff, received a stage 4 mantle cell lymphoma diagnosis in 2021. After undergoing multiple chemotherapy rounds and achieving remission, his cancer unfortunately returned in July 2024.
"Fortunately, I was referred for CAR T-cell therapy and began treatment during autumn 2024," Mr Madley recounted. "By March 2025, I received the wonderful news that I was in remission, a status I maintain today. I have resumed working as a consultant three to four days weekly, walk my dog most mornings, play golf regularly and generally enjoy my pre-illness lifestyle. Learning about Nice's decision to withdraw this treatment feels truly unbelievable, provoking sadness, profound disappointment and anger. This therapy is actively prolonging lives like mine—without it, my situation would be unimaginably different."
Organisational Responses and Future Considerations
Ropinder Gill, chief executive at Lymphoma Action, reported that the charity has fielded numerous enquiries from concerned and anxious community members following Nice's announcement. "Substantial distress has emerged regarding the potential removal of this treatment option," she added, highlighting the emotional toll on patients and families.
A spokesperson for Nice confirmed that the organisation welcomes the formal appeal and will carefully consider all points raised through established procedures. They emphasised that any patient who has already commenced Tecartus treatment will be permitted to complete their therapeutic course. "We fully recognise this decision will prove deeply disappointing for mantle cell lymphoma patients and their families, and we acknowledge the distress it has caused," the spokesperson stated. "Other treatment alternatives remain available, and Nice is currently evaluating two additional therapies—sonrotoclax and acalabrutinib—which may offer future hope for this patient population."
The charity coalition has collectively characterised Nice's recommendation as a "backward step for NHS care," underscoring their commitment to ensuring medical advances in blood cancer treatment translate into practical options for patients in clinical settings. The appeal process now moves forward as stakeholders await further developments regarding this critical healthcare decision.





