Entertainment
Madeline Cash's 'Lost Lambs' Catapults Her to Literary Stardom Amid Rumours
Madeline Cash's debut novel 'Lost Lambs' has been hailed as the year's brightest literary achievement, praised for its witty take on American dysfunction, though she faces nepotism rumours.
Sports
Arsenal's Kai Havertz Ruled Out of Tottenham Clash with New Injury Setback
Arsenal forward Kai Havertz will be absent for the crucial north London derby against Tottenham after sustaining a fresh injury, compounding existing long-term fitness concerns at the club.
Politics
Halle Berry's $200K Engagement Ring Steals Spotlight at Crime 101 Premiere
Halle Berry showcased her stunning engagement ring from Van Hunt at the Crime 101 premiere, with experts valuing the Art Deco piece between $120,000 and $200,000.
Crime
Lammy Announces Mandatory Knife Crime Support Plan for Children
Deputy PM David Lammy unveils mandatory support plans for children caught with knives in England and Wales, following stabbings at a London school.
Health
Weather
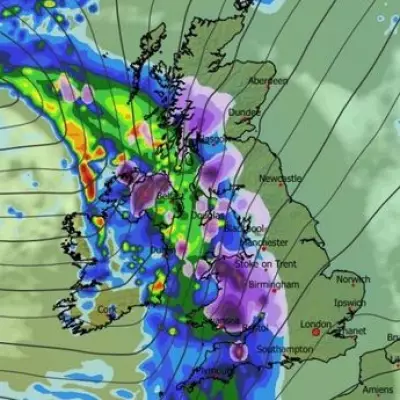
Twin Blizzards to Bury UK Cities with 19 Inches of Snow
Advanced weather maps reveal two major blizzards will hit the UK, bringing up to 19 inches of snow to cities like London and Birmingham, with intense flurries expected.
Cyclone Gezani Kills 20 in Madagascar, Causes Widespread Damage
Tropical Cyclone Gezani has struck Madagascar, resulting in at least 20 fatalities and severe infrastructure damage, with thousands evacuated and warnings of further storms ahead.
Snow Warning for 26 UK Cities on Thursday and Friday
The Met Office issues yellow warnings as snow is forecast to sweep from Scotland southwards, with up to 10cm expected in some areas and travel disruption likely.
UK's Record 42-Day Rain Deluge: Causes and Forecast
The UK faces a record-breaking 42 consecutive days of rainfall, with forecasters warning of continued downpours and flooding. A stalled low-pressure system is to blame.
UK Cold Weather Alert as Temperatures Drop to -4C
The UK Health Security Agency has issued a yellow cold weather health alert for large parts of the UK from Friday to Monday, warning of risks to vulnerable people as temperatures fall below zero.
Tech
Get Updates
Subscribe to our newsletter to receive the latest updates in your inbox!
We hate spammers and never send spam
Environment
Tuesday Test: Can You Answer These UK Sports Questions?
Test your knowledge of British sports with our Tuesday quiz featuring 100 questions about football, cricket, and rugby. Perfect for sports enthusiasts and trivia lovers.