In a devastating blow to brain cancer patients across the UK, the National Health Service has rejected a pioneering new treatment that could significantly extend lives and delay disease progression.
The decision by NICE (National Institute for Health and Care Excellence) means thousands of patients with specific low-grade gliomas will be denied access to vorasidenib, a drug hailed as the first major breakthrough in brain cancer treatment in decades.
Revolutionary Treatment Blocked
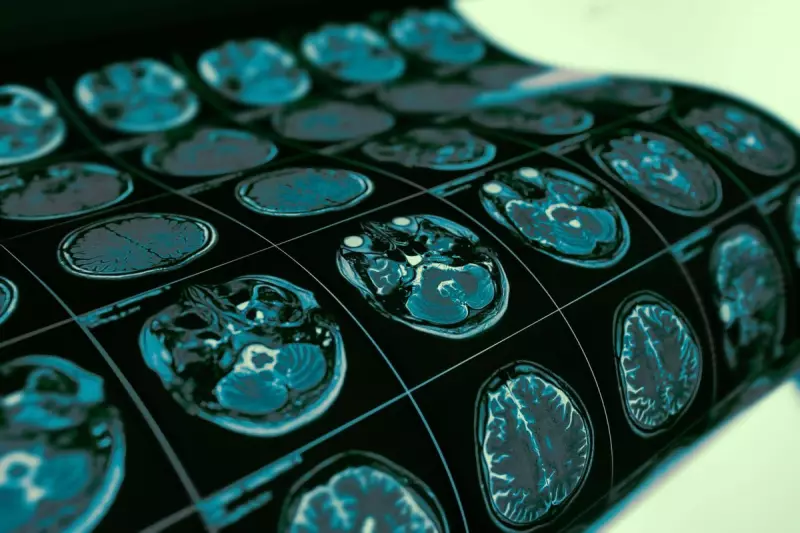
Vorasidenib represents a monumental advancement in neuro-oncology, specifically targeting IDH-mutant diffuse gliomas. Clinical trials demonstrated remarkable results, with the drug reducing the risk of disease progression or death by an impressive 61%.
Patients receiving the treatment experienced significantly delayed time until their next required intervention, potentially offering years of additional quality life without the need for aggressive treatments like chemotherapy and radiation.
Cost Concerns Override Patient Benefits
The rejection stems from NICE's assessment that vorasidenib doesn't represent sufficient value for money for the NHS, despite its proven clinical effectiveness. This decision highlights the ongoing tension between revolutionary medical advances and the financial constraints facing Britain's healthcare system.
Cancer charities and patient advocacy groups have expressed profound disappointment, noting that the UK now risks falling behind other developed nations where the drug is expected to receive approval.
Patient Stories Highlight Human Cost
For individuals living with these slow-growing but ultimately fatal brain tumours, the decision represents more than just bureaucratic red tape. It means continued uncertainty and the prospect of undergoing more invasive treatments sooner than might otherwise be necessary.
Many patients and their families had pinned their hopes on vorasidenib, which offered the possibility of managing their condition as a chronic disease rather than an immediate death sentence.
What Happens Next?
There remains a possibility that the manufacturer, Servier, could reach a compromise with NHS England through a commercial arrangement. However, for now, British patients face the bitter reality of watching other countries access a treatment that remains out of their reach.
The decision underscores the urgent need for reforms in how the NHS evaluates and funds innovative cancer treatments, particularly for conditions where therapeutic options have remained stagnant for years.