The US Food and Drug Administration (FDA) has issued a critical safety alert, urging patients to immediately stop using certain glucose monitoring devices after they were linked to seven fatalities and more than 700 serious injuries.
Critical Malfunction in Abbott Devices
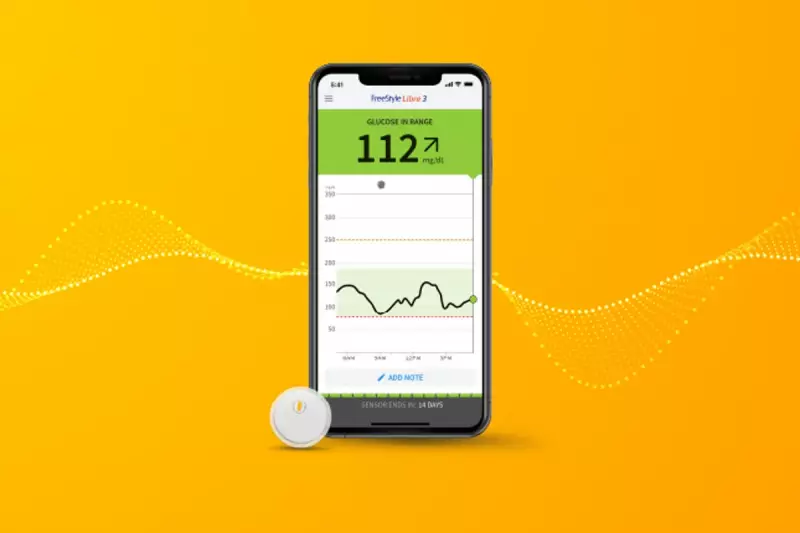
The warning concerns specific models of the FreeStyle Libre 3 and FreeStyle Libre 3 Plus continuous glucose monitors (CGMs), manufactured by Abbott Diabetes Care. According to the company, sensors in these devices "may provide incorrect low glucose readings."
Abbott stated that the malfunction was traced to a single production line among several. The firm reported a total of 736 severe adverse events globally, with 57 occurring within the United States. While the seven reported deaths are potentially associated with the device issue, the company confirmed that none of those fatalities happened in the US.
Immediate Health Risks for Diabetes Patients
The FDA has emphasised the grave danger posed by the inaccurate readings. "If undetected, incorrect low glucose readings over extended periods of time could lead to incorrect treatment decisions," the agency stated.
This could include people with diabetes consuming excessive carbohydrates or dangerously delaying necessary insulin doses. "These decisions may pose serious health risks, including potential injury or death," the FDA's warning concluded.
Approximately three million devices are believed to be affected, though Abbott notes many may have already expired or been used. The company insists it has resolved the root cause and does not anticipate significant supply disruptions.
What Patients Must Do Now
The FDA and Abbott are urging all users of FreeStyle Libre 3 or FreeStyle Libre 3 Plus systems to take immediate action:
- Check the model number and unique device identifiers on your sensor.
- Visit the dedicated website www.FreeStyleCheck.com to verify if your sensor is impacted.
- "Immediately discontinue use and dispose of" any affected sensors, per FDA instructions.
Abbott has committed to replacing any potentially affected sensors free of charge. The scale of this alert is significant, given that the Centers for Disease Control and Prevention estimates about 1 in 10 people in the US (38.4 million) have diabetes, which was the eighth leading cause of death in the country as of 2021.





