For countless children across the United Kingdom and beyond, receiving a first prescription for glasses is a common childhood rite of passage. However, a groundbreaking new development means that spectacles could now do more than just correct vision—they might actively help preserve it for the long term.
A New Frontier in Treating Childhood Myopia
In a significant move for paediatric eye care, the US Food and Drug Administration (FDA) approved a novel type of spectacle lens in September 2025 designed to slow the progression of nearsightedness, or myopia, in children aged 6 to 12. While this technology has been available in Europe and Asia, its official clearance marks a major step for treatment options in the US, with implications for global practice.
Myopia, where close objects are clear but distant ones appear blurry, is rising sharply worldwide. Experts link this trend to increased time spent indoors focusing on screens, books, and other near-range objects. In the US, 30% to 40% of children develop myopia by the end of secondary school, according to Dr. Michael Repka, a professor at Johns Hopkins School of Medicine.
"The traditional approach was simply: 'Your child needs to wear glasses and they’ll live with it,'" Dr. Repka explained. "We'd tell parents it would be lifelong and likely worsen over the next few years." This new technology fundamentally changes that conversation.
How the Innovative Lenses Work
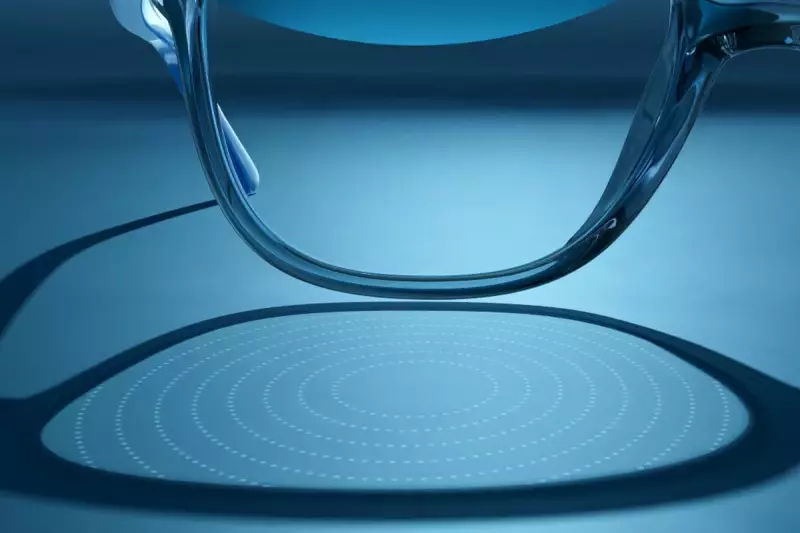
The specialised glasses, marketed under the brand Essilor Stellest by EssilorLuxottica, employ a unique optical design. The lenses feature 11 concentric rings filled with tiny raised dots that refocus light onto the retina in a specific manner. This is believed to slow the elongation of the eyeball, which is the physical change that causes worsening myopia and increases the risk of serious future eye problems.
The FDA's approval was based on company data showing a remarkable 70% reduction in myopia progression after two years of use. Furthermore, children wearing the lenses demonstrated a 50% reduction in eye lengthening over the same period.
The potential long-term benefits are substantial. Severe myopia increases the risk of conditions like cataracts, glaucoma, and retinal detachment, which can lead to blindness. "Now we have a way to slow that down," said Honolulu-based paediatric ophthalmologist Dr. Rupa Wong. "Maybe we can prevent kids from having that really elongated eye that puts them at risk for blindness."
Availability, Cost, and Comparison to Other Treatments
The suggested retail price for the lenses is $450. Major US vision insurance providers are expected to cover them for children who meet the prescribing criteria, a model that may influence coverage discussions in the UK and other markets.
Previously, the only other FDA-approved product to slow myopia was MiSight daily disposable contact lenses, cleared in 2019 for children aged 8 to 12. However, the new spectacles offer a crucial alternative. "A lot of people might be hesitant to put a child as young as 8 in contact lenses, so the glasses offer a really nice alternative," noted Dr. Wong.
Some doctors have also prescribed off-label medicated eye drops, though these lack formal FDA approval for this purpose. Under the FDA decision, the new lenses can be prescribed to any child with myopia within the 6-12 age range. Reported side effects were minimal, with some children experiencing temporary visual disturbances like halos.
It is important to note that the studies reviewed by the FDA were conducted in Asia. Dr. Repka, who is leading a US-based study supported by the National Institutes of Health, stated, "I think before it becomes widely used, we will need some data in the United States" to confirm the results. This call for further independent research is a standard and prudent step in adopting any new medical technology.
This approval represents a pivotal shift from merely correcting myopia to actively managing its progression, offering hope for better long-term eye health for a generation of children.





