In a landmark decision for cancer care in the UK, a pioneering treatment that allows some rectal cancer patients to avoid major surgery has been approved for use on the NHS. The National Institute for Health and Care Excellence (Nice) has recommended the Papillon procedure, a move hailed as a "great victory" for patient choice and quality of life.
What is the Papillon Treatment?
Papillon is a specialised form of brachytherapy that targets small rectal tumours with remarkable precision. The technique involves inserting a small X-ray tube directly through the anus and into the rectum. This allows clinicians to deliver low-energy radiation directly to the tumour site, minimising damage to surrounding healthy tissue.
Nice has recommended the treatment for fit patients with tumours measuring 3cm or less who either choose not to undergo surgery or are deemed too high-risk for an operation. The approval also extends to patients with larger tumours if other treatments successfully shrink them to the 3cm threshold.
Avoiding Surgery and Improving Lives
The most significant benefit of the Papillon procedure is its potential to allow patients to bypass radical surgery. Traditional surgery for rectal cancer often requires the creation of a stoma – an opening in the abdomen to divert waste into an external bag. Nice states that avoiding this procedure "substantially improves" a patient's quality of life.
Professor Sun Myint, the consultant in clinical oncology at the Clatterbridge Cancer Centre in Merseyside who pioneered Papillon, expressed his delight. "I have been treating patients with this therapy for more than 33 years, which equates to about 3,000 people," he said. "This decision is a great victory for patients who will now have a choice for the treatment they prefer."
Proven Success and Patient Stories
The approval follows compelling evidence from clinical trials, including the Opera trial led by Prof Myint. The five-year study found that Papillon helped preserve organs 93% of the time in eligible rectal cancer cases.
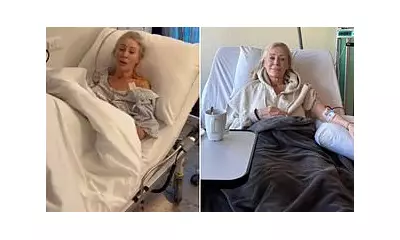
Sharon Price, a 45-year-old NHS worker from Newcastle-under-Lyme, was successfully treated as part of this study. Faced with the prospect of life with a permanent stoma, she described the offer of the Papillon trial as a lifeline. "That was devastating – I was just too young to have to go through that," she said. "I was offered the chance to join the clinical trial, and I decided to do it immediately."
Dr Caroline Brammer, medical director at The Clatterbridge Cancer Centre, highlighted the wider benefits. "This development will help reduce surgical waiting lists and costs to the NHS and improve quality of life for many patients with rectal cancer," she said.
With more than 41,000 new cases of colorectal cancer diagnosed annually in the UK, this new non-surgical treatment option represents a major step forward. Prof Myint, now 77, sees the Nice recommendation as a career capstone: "I feel that I have done my job and I can now hang up my gloves, but not until this treatment is embedded as the standard of care in the NHS and across the world."