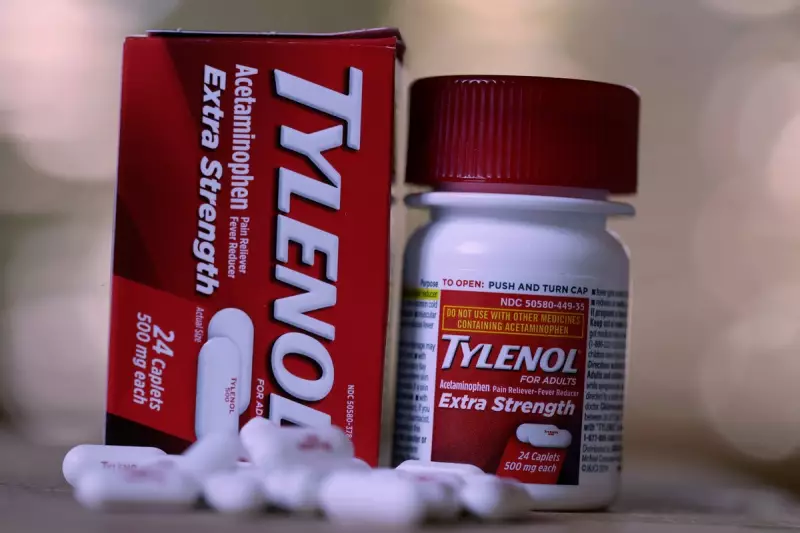
A major pharmaceutical storm is brewing across the Atlantic that could have significant implications for British families and healthcare providers. Thousands of American families are pursuing legal action against manufacturers of Tylenol—known as paracetamol in the UK—alleging that prenatal use of the common painkiller caused autism spectrum disorder (ASD) in their children.
The American Legal Battle
In the United States, approximately 400 lawsuits have been consolidated into multidistrict litigation against Kenvue, the consumer health spinoff of Johnson & Johnson. The plaintiffs claim the company failed to adequately warn pregnant women about potential neurological risks to their unborn children.
While American courts grapple with these complex cases, British health experts are watching closely. The allegations centre on acetaminophen (paracetamol), one of the world's most commonly used medications during pregnancy.
The Scientific Debate
The controversy stems from several scientific studies suggesting a potential correlation between prenatal paracetamol exposure and neurodevelopmental issues. A landmark 2021 consensus statement signed by 91 scientists and doctors called for precautionary labelling, noting that prolonged use during pregnancy might increase autism and ADHD risks by approximately 30%.
However, the scientific community remains divided. Many medical organisations maintain that occasional, short-term use remains the safest option for pain relief during pregnancy when necessary.
What This Means for UK Families
For British parents, the US lawsuits raise important questions about medication safety. The NHS currently states that paracetamol is "usually safe" during pregnancy, but recommends using the lowest effective dose for the shortest possible time.
Medical professionals emphasise that untreated pain and fever during pregnancy also carry risks, creating a complex balancing act for expectant mothers and their doctors.
Pharmaceutical Industry Response
Kenvue, the defendant in the US cases, maintains that Tylenol has over 50 years of clinical evidence supporting its safety profile. The company argues that the lawsuits are not supported by science and that labelling already advises pregnant women to consult their doctors.
The outcome of these American legal battles could potentially influence global pharmaceutical labelling practices and regulatory approaches.
As research continues, British health authorities face increasing pressure to provide clear, evidence-based guidance to help pregnant women make informed decisions about pain management.