Pharmaceutical titan Pfizer is facing a monumental legal battle as more than 2,100 women across the United States file lawsuits claiming a popular contraceptive injection caused them to develop debilitating brain tumours. The first trial in the sprawling litigation is now scheduled for 7 December 2026.
The Allegations and the Impending Trial
The women allege that Pfizer failed to adequately warn them that the birth control shot Depo-Provera was linked to a significantly increased risk of developing meningiomas—benign tumours that grow in the membranes surrounding the brain and spinal cord. Lawyers representing the plaintiffs revealed exclusively to the Daily Mail that the trial date has been set, marking a critical juncture in the case.
Virginia Buchanan, a partner at Levin Papantonio law firm and a court-appointed co-chair of the Plaintiffs’ Executive Committee, stated the new warning label added by Pfizer last month was "a long time coming" and "long overdue." She urged any woman diagnosed with a meningioma who has used Depo-Provera to contact legal counsel "sooner rather than later" to join the litigation.
Understanding the Risk and the Science
Depo-Provera is an injectable contraceptive used by an estimated two million women annually. It works by delivering a synthetic hormone called progestin to prevent pregnancy. Recent scientific studies have sounded alarm bells, however. A landmark 2024 study in the British Medical Journal found that using Depo-Provera for 12 months or more was associated with a 5.6-fold increased risk of requiring surgery for a meningioma.
Another 2025 study in Expert Opinion on Drug Safety reported a 3.5-fold increased risk of developing an intracranial meningioma with long-term use compared to birth control pills. Experts theorise that the synthetic progestin may overstimulate receptors in the meninges, potentially leading to cell mutation and tumour growth.
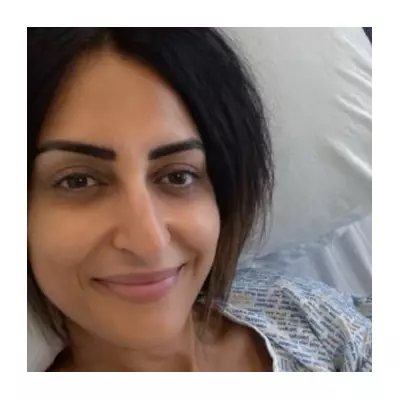
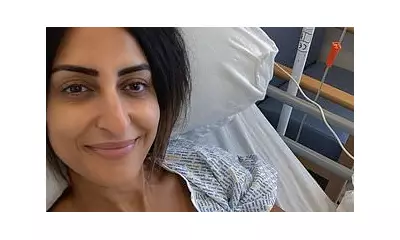
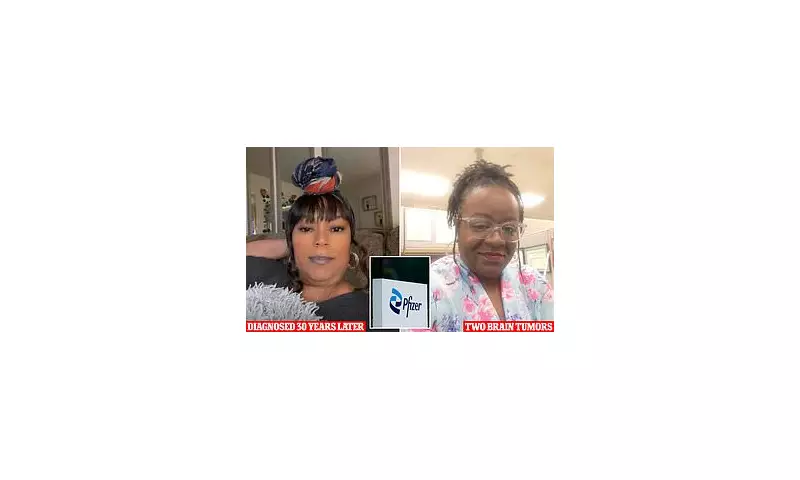
While meningiomas are typically non-cancerous, they can cause severe, life-altering symptoms as they slowly expand. Plaintiffs have reported impaired vision, hearing, and smell, seizures, memory loss, and debilitating headaches. The slow growth often means symptoms are not linked to the contraceptive use for years or even decades.
A Growing Wave of Litigation and Its Implications
The number of claimants has surged five-fold since May 2024, and Buchanan expects more to come forward, especially following the updated FDA warning label. "We have had a significant uptick in the filings," she said, emphasising the case's role as a critical women's health issue.
The lawsuits contend that Pfizer knew of the risks but did not warn consumers or promote safer alternatives. "The basis of this lawsuit is there should have been a warning," Buchanan asserted. "These women... can't go back and undo... what they've been exposed to, but certainly women going forward can."
The initial trial will involve a single plaintiff, with trials for four additional plaintiffs scheduled every 60 days thereafter. Pfizer, which sought the label change but has denied wrongdoing, may choose to settle before trial. Law firms are also investigating potential class actions in other jurisdictions, including Europe, Australia, South Africa, and Canada.
For the thousands of women affected, the case represents a fight for accountability and awareness, hoping to prevent others from facing similar "real and life-changing" consequences.