In a significant development that has sent shockwaves through the pharmaceutical industry, Indian authorities have made multiple arrests following an international health scare involving allegedly toxic cough syrup.
The Arrests and Investigation
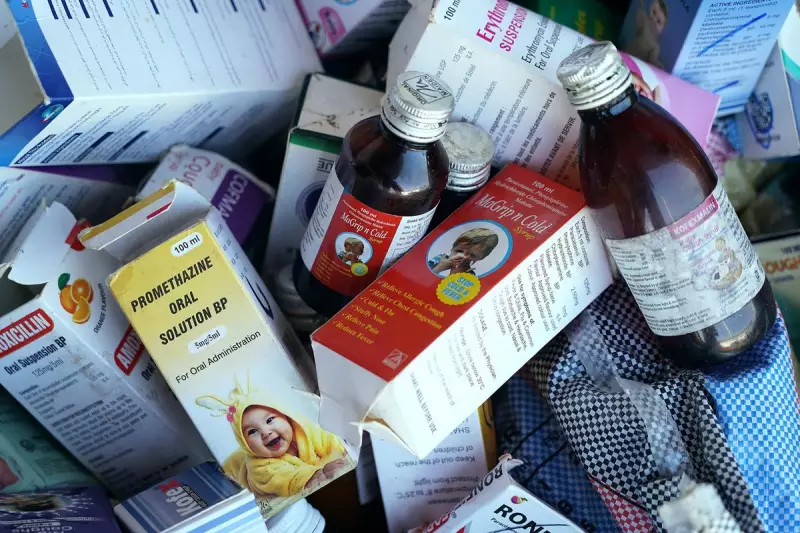
Three senior executives from Sresan Pharmaceutical, based in New Delhi, have been taken into custody as part of an ongoing criminal investigation. The arrests come after laboratory tests revealed dangerous contaminants in cough syrup manufactured by the company that was subsequently exported to Cameroon.
The detained individuals include the company's director, a production supervisor, and a quality control manager, all facing serious charges related to the production and distribution of substandard medicines.
International Health Crisis
The controversy emerged when Cameroonian health officials reported multiple child fatalities linked to the consumption of the cough syrup. Laboratory analysis conducted by both Cameroonian and international health agencies detected unacceptable levels of diethylene glycol and ethylene glycol - toxic substances commonly used in antifreeze that can cause acute kidney failure and death when ingested.
"This represents a catastrophic failure of quality control and regulatory oversight," stated a spokesperson from India's Central Drugs Standard Control Organisation.
Global Pattern of Concern
This incident marks the latest in a series of international scandals involving Indian-manufactured cough syrups. In recent years, similar contamination issues have been linked to child deaths in Gambia, Uzbekistan, and Indonesia, raising serious questions about regulatory enforcement within India's massive pharmaceutical export industry.
The World Health Organization has issued multiple medical product alerts concerning Indian-made syrups, highlighting an urgent need for strengthened quality assurance protocols.
Industry Response and Regulatory Action
Indian pharmaceutical authorities have launched a comprehensive review of manufacturing practices across the sector. "We cannot allow the reputation of India's pharmaceutical industry to be compromised by a few negligent manufacturers," declared a senior health ministry official.
The investigation continues as authorities examine how the contaminated products passed quality checks and were cleared for export. Additional arrests and regulatory actions are anticipated as the probe expands to include supply chain partners and testing laboratories.