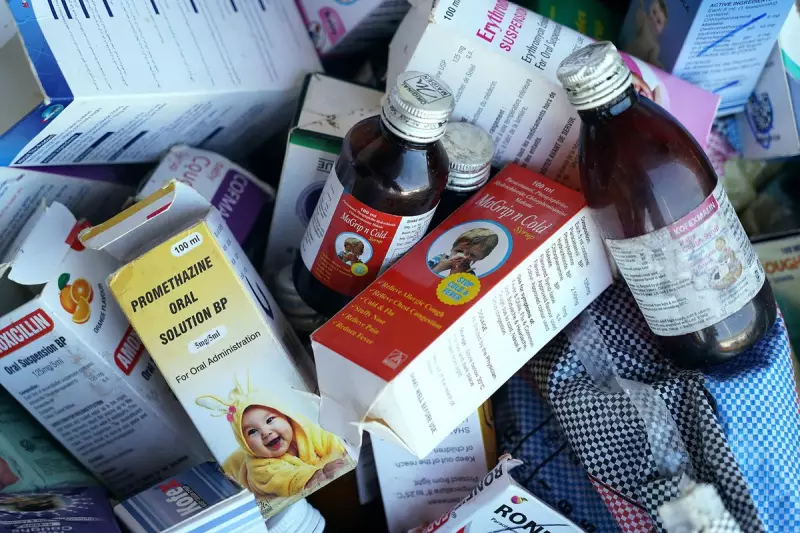
A devastating health crisis has unfolded in central India after eight young children lost their lives to suspected poisoning from contaminated cough syrup. The tragedy occurred in the Umaria district of Madhya Pradesh, sending shockwaves through the local community and raising urgent questions about pharmaceutical safety standards.
The Heartbreaking Timeline
According to local authorities, the children began showing severe symptoms after consuming cough syrup purchased from a neighbourhood pharmacy. Medical officials confirmed the victims ranged in age from very young children to early teens, with the first fatalities reported earlier this month.
Dr Ritesh Chawla, the district's chief medical officer, revealed: "We've sent samples for laboratory testing to determine the exact cause of contamination. Initial investigations point toward possible chemical poisoning from the medicinal syrup."
Immediate Response and Investigation
Local police have launched a comprehensive investigation, questioning the pharmacist who sold the medication and tracing the supply chain back to the manufacturer. The pharmacy in question has been temporarily sealed while authorities conduct their probe.
Health officials have issued urgent warnings to local residents, advising them to immediately discontinue use of the suspected syrup and seek medical attention if they experience any unusual symptoms.
Broader Implications for Pharmaceutical Safety
This tragic incident echoes similar cases that have plagued India's pharmaceutical industry in recent years. Just last year, cough syrups manufactured in India were linked to child deaths in The Gambia and Uzbekistan, prompting global concern about quality control measures in drug manufacturing.
The Madhya Pradesh tragedy raises critical questions about:
- Quality control protocols in pharmaceutical manufacturing
- Regulatory oversight of medical products
- Supply chain transparency for medicines
- Emergency response systems for medical emergencies
As investigations continue, families in Umaria district mourn their devastating losses while health authorities work to prevent further tragedies. The case serves as a stark reminder of the vital importance of rigorous safety standards in pharmaceutical production and distribution.