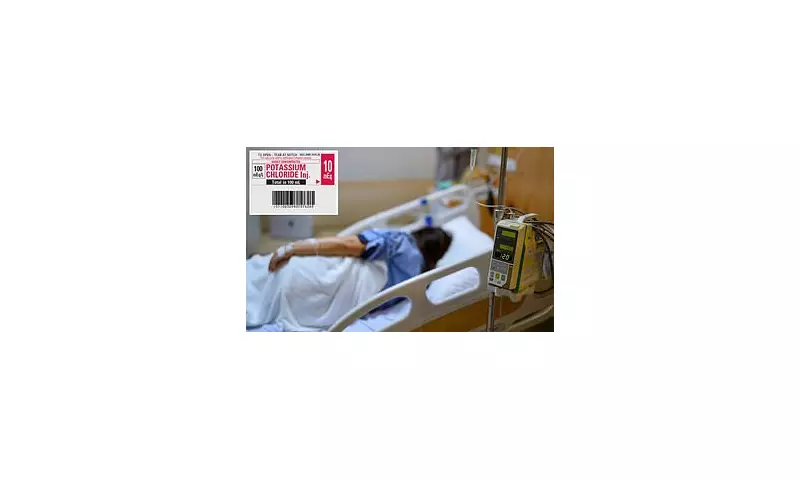
In a startling development that has sent shockwaves through the medical community, health authorities have issued an urgent recall of a potentially life-threatening medication error. Thousands of mislabeled tablets have been distributed to pharmacies across the United Kingdom, putting vulnerable patients at serious risk.
The Critical Error
The US Food and Drug Administration (FDA) has sounded the alarm after discovering that potassium chloride tablets, essential for treating the dangerous condition hypokalemia, were incorrectly packaged as carbamazepine – a medication used for epilepsy and nerve pain.
This catastrophic labelling mistake could have fatal consequences for patients suffering from low potassium levels, a condition that can lead to severe heart rhythm abnormalities and muscle weakness.
What Patients Need to Know
The affected medication bears the following identifying information:
- Product: Potassium Chloride Extended-release Tablets USP, 750mg (10 mEq)
- Lot Number: 231018
- Expiration Date: 10/2025
- UPC Code: 1074681001
Immediate Health Risks
Patients who accidentally take carbamazepine instead of their prescribed potassium medication face dual dangers:
- Untreated hypokalemia leading to potential cardiac arrest
- Unintended side effects from unnecessary carbamazepine exposure
"This is precisely the type of medication error that keeps healthcare professionals awake at night," said a senior NHS pharmacist who wished to remain anonymous. "The consequences of such a mix-up could be devastating for patients relying on this medication to maintain stable heart function."
Urgent Action Required
Health officials are urging anyone prescribed potassium chloride tablets to:
- Immediately check their medication packaging
- Contact their pharmacist if they suspect they have the recalled product
- Do not stop taking any medication without consulting their GP
- Seek immediate medical attention if they experience unusual symptoms
The manufacturer, Marksans Pharma, has initiated the voluntary recall while working closely with regulatory authorities to investigate how this dangerous labelling error occurred.
This incident serves as a stark reminder of the critical importance of medication safety protocols and the potentially catastrophic consequences when they fail.





