In a monumental step forward for global HIV prevention, a revolutionary long-acting injection that provides six months of protection against the virus is set to become available in 120 lower-income countries by 2027. The groundbreaking agreement promises to transform the landscape of AIDS prevention in regions hardest hit by the epidemic.
A New Era in HIV Prevention
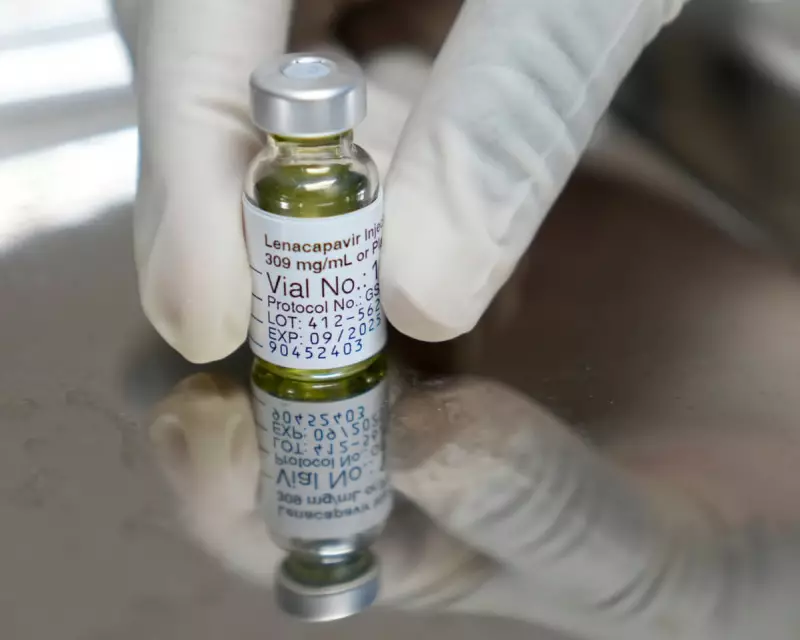
The drug, lenacapavir, represents the most significant advancement in HIV prevention in decades. Unlike daily pills that require consistent adherence, this innovative injection needs administering just twice yearly, dramatically simplifying prevention for millions at risk.
Developed by Gilead Sciences, the pharmaceutical giant behind leading HIV treatments, lenacapavir works by disrupting the HIV virus's capsid, the protein shell that protects its genetic material. This novel mechanism makes it effective against strains that have developed resistance to older medications.
Landmark Access Agreement
The accessibility breakthrough comes through a pioneering licensing agreement between Gilead and the Medicines Patent Pool, a United Nations-backed organisation. This arrangement will allow generic manufacturers to produce affordable versions specifically for 120 eligible countries, home to the majority of people living with or at risk of HIV worldwide.
This initiative marks the first time a long-acting HIV prevention medication will be made available through such a comprehensive access programme, potentially preventing thousands of new infections annually.
Addressing Global Inequalities
Current HIV prevention methods, while effective, face significant challenges in resource-limited settings. Daily pill regimens can be difficult to maintain consistently, and access to healthcare facilities varies widely. The twice-yearly injection model could overcome these barriers, offering discreet, convenient protection that doesn't depend on daily routines.
Countries across sub-Saharan Africa, Southeast Asia, and other regions with high HIV prevalence stand to benefit enormously. The timing is particularly crucial as global HIV prevention efforts have stalled in recent years, with approximately 1.3 million new infections occurring annually worldwide.
What Happens Next?
The rollout timeline is already taking shape:
- 2025-2026: Regulatory approvals and manufacturing preparations
- 2027: Initial availability in eligible countries
- 2028 onwards: Scaling up to full distribution
While the injection is currently approved in the US and European Union for treating established HIV infections, its prevention application is still under regulatory review. However, clinical trials have demonstrated exceptional effectiveness, showing 100% efficacy in preventing HIV acquisition among trial participants.
This development represents hope for millions and signals a potential turning point in the four-decade fight against AIDS. As one global health expert noted, it's not just a medical breakthrough but a commitment to health equity that could alter the course of the epidemic for generations to come.