In a significant breakthrough for obesity treatment, pharmaceutical giant Eli Lilly has unveiled promising results for a new weight loss pill that could transform how the condition is managed in the UK.
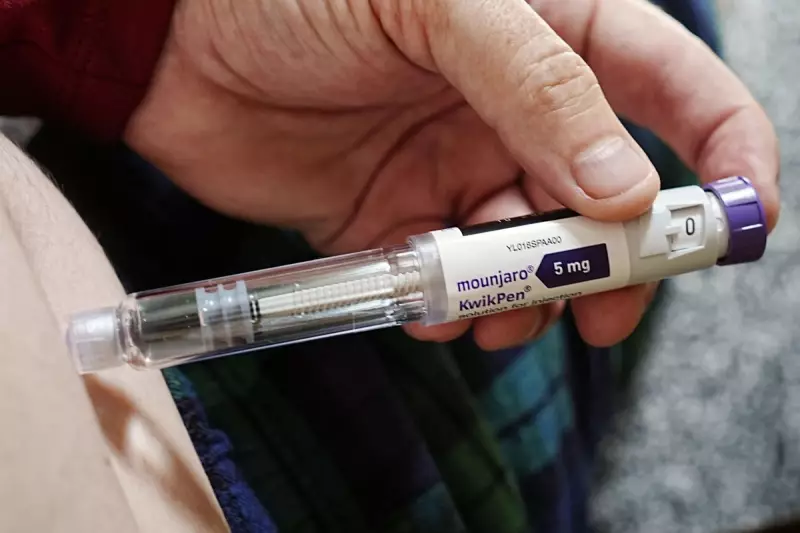
The drug, orforglipron, has shown impressive efficacy in a recent clinical trial, positioning itself as a potent oral alternative to the company's own injectable treatment, Mounjaro (tirzepatide).
A Game-Changer in Obesity Medication
The Phase 2 trial demonstrated that participants taking orforglipron achieved substantial weight reduction over a 36-week period. Patients on the highest dose experienced up to a 14.7% decrease in body weight, showcasing the pill's potential as a powerful tool against obesity.
This development is particularly noteworthy as it offers patients a convenient oral option, potentially overcoming the hesitation some feel towards injectable treatments like semaglutide (Wegovy) or tirzepatide (Mounjaro).
How Orforglipron Works
Orforglipron functions as a GLP-1 receptor agonist, mimicking the effects of natural hormones that regulate appetite and insulin secretion. By activating these receptors, the drug helps patients feel fuller for longer, reduces food intake, and improves blood sugar control.
The oral formulation represents a significant advancement in making this class of medications more accessible and user-friendly for the millions struggling with weight management issues.
Addressing a Growing Health Crisis
The introduction of orforglipron comes at a critical time for the UK, where obesity rates continue to pose serious public health challenges. With approximately two-thirds of adults in England classified as overweight or obese, effective and accessible treatment options are in high demand.
This new pill could potentially reduce the burden on the NHS by helping to prevent obesity-related conditions such as type 2 diabetes, heart disease, and certain cancers.
Safety and Next Steps
While the trial results are encouraging, researchers noted that gastrointestinal side effects were common among participants, though these were generally mild to moderate and decreased over time.
Eli Lilly is now progressing with Phase 3 trials to further evaluate the drug's long-term safety and effectiveness. If successful, orforglipron could be submitted for regulatory approval in the coming years, potentially offering a new weapon in the fight against obesity.
The development of orforglipron underscores the pharmaceutical industry's growing focus on addressing the global obesity epidemic through innovative medical solutions that cater to patient preferences and needs.